Article Presented By Kingston National Bank… (Waverly) – The second annual Fluor-BWXT Diversity Council’s Dr....
Uncategorized
Article Presented By Pickaway Ross CTC… (Chillicothe) – The Rulon Center will hold an Open...
Article Presented By Classic Brands… (Columbus) – The Division of State Fire Marshal announces the appointment...
Article Presented By Accurate Heating, Cooling & Plumbing… The Southern Ohio Christian Conference will be...
Article Presented By Scioto Valley Dumpsters, LTD… (Chillicothe) – Ohio University Chillicothe accepting applications for the...
Presented by McDonald’s, I’m Lovin’ It! The Adena Warriors needed every weapon in their arsenal...
Presented By Rathkamp Financial
Presented By McDonald’s, I’m Lovin’ It! As we look toward fall, we know blood donor...
Presented By Hometown-Motors, Inc. (Washington D.C.) — The U.S. Food and Drug Administration has given...
Presented By Classic Brands (CHILLICOTHE) – Calling it a “game changer” in patient care across southern...
Presented by Hometown-Motors, Inc. For a fourth consecutive season, the Litter Media cameras will be...
Presented By Hometown-Motors, Inc.
Presented By Classic Brands (Chillicothe) –– The Chillicothe VA Medical Center and its Community Based Outpatient...
Presented By Atomic Speedway UPDATED 8/17/21 Area health departments are administering third COVID-19 Pfizer &...
Presented By Rathkamp Financial (Chillicothe) — Enjoy ice cream, cakes, lemonade, and other refreshments for...
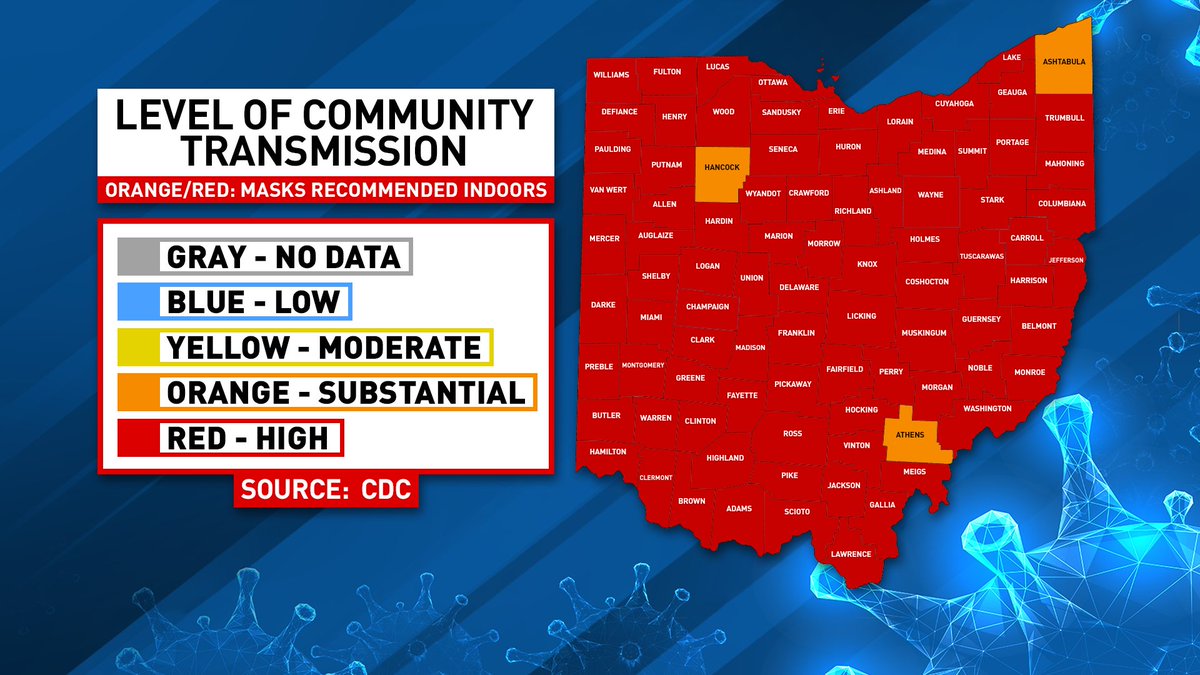
Presented By Classic Brands 85 of Ohio’s 88 counties have moved to the highest level...
Presented By Hometown-Motors, Inc. The 39th Annual Foothills Art Festival will have a new exhibition...
Presented By Classic Brands The Pike County Fairgrounds will receive an upgrade thanks to a...
Presented By Atomic Speedway (Ross County Fairgrounds) — The Junior Fair Livestock Sale Committee has...
Presented By Rathkamp Financial The Hillsboro Indians held home course advantage Wednesday, winning Match #1...
Presented By McDonald’s, I’m Lovin’ It! (CHILLICOTHE) – While the physical presence of the Adena Orthopedic...
Presented By Hometown-Motors, Inc. (Lafayette) — Going 4 hours in the longest game of the...
Presented By Classic Brands Corn growers from around the region are invited to the Annual...
Presented By Atomic Speedway The Ross County Fair will close its 77th edition Saturday night,...
Article Presented By Rathkamp Financial (Waverly) — The Community Action Committee of Pike County (CAC)...
Presented By Classic Brands (Chillicothe) — A revision has been made by the City of...
Presented By McDonald’s, I’m Lovin’ It! The USDA Farm Service Agency is taking applications for...
Presented By Hometown-Motors, Inc.
Presented By Classic Brands The Pike County community is encouraged to attend a Saturday, August...
Presented By Atomic Speedway (Chillicothe) — Comedy comes to the Ross County Fair Bandstand on...